Ubiquinone
Ubiquinone is the INCI name for CoQ10.
This article is missing information about biological function (weight too low compared to dietary), need a section with links to Q cycle and Complex III at minimum. (September 2022) |
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10.
| |
| Names | |
|---|---|
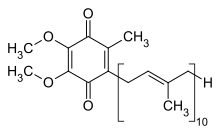
| Preferred IUPAC name
2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-Decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaen-1-yl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | |
| Other names
Ubiquinone, ubidecarenone, coenzyme Q, CoQ10, /ˌkoʊˌkjuːˈtɛn/, CoQ, Q10, vitamin Q
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.590 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C59H90O4 | |
| Molar mass | 863.365 g·mol−1 |
| Appearance | yellow or orange solid |
| Melting point | 48–52 °C (118–126 °F; 321–325 K) |
| insoluble | |
| Pharmacology | |
| C01EB09 (WHO) | |
| Related compounds | |
Related quinones
|
1,4-Benzoquinone Plastoquinone Ubiquinol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It is a 1,4-benzoquinone, where Q refers to the quinone chemical group and 10 refers to the number of isoprenyl chemical subunits in its tail. In natural ubiquinones, the number can be anywhere from 6 to 10. This family of fat-soluble substances, which resemble vitamins, is present in all respiring eukaryotic cells, primarily in the mitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, which generates energy in the form of ATP. Ninety-five percent of the human body's energy is generated this way. Organs with the highest energy requirements—such as the heart, liver, and kidney—have the highest CoQ10 concentrations.
There are three redox states of CoQ: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol). The capacity of this molecule to act as a two-electron carrier (moving between the quinone and quinol form) and a one-electron carrier (moving between the semiquinone and one of these other forms) is central to its role in the electron transport chain due to the iron–sulfur clusters that can only accept one electron at a time, and as a free-radical–scavenging antioxidant.









