Tocopheryl Acetate
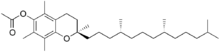
Tocopheryl acetate, also known as vitamin E acetate, is a synthetic form of vitamin E. It is the ester of acetic acid and α-tocopherol. Tocopheryl acetate is often used in dermatological products such as skin creams. It is not oxidized and can penetrate through the skin to the living cells, where about 5% is converted to free tocopherol.
α-Tocopheryl acetate (alpha-tocopherol acetate), also known as vitamin E acetate, is a synthetic form of vitamin E. It is the ester of acetic acid and α-tocopherol.
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2R)-2,5,7,8-Tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydro-2H-1-benzopyran-6-yl acetate | |
| Other names
α-Tocopherol acetate
Vitamin E acetate | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.369 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C31H52O3 | |
| Molar mass | 472.743 g/mol |
| Appearance | pale yellow, viscous liquid |
| Melting point | –27.5 °C |
| Boiling point | 240 °C decays without boiling |
| insoluble | |
| Solubility | soluble in acetone, chloroform, diethyl ether; poorly soluble in ethanol |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The U.S. Centers for Disease Control and Prevention says that vitamin E acetate is a very strong culprit of concern in the 2019 outbreak of vaping-associated pulmonary injury (VAPI), but there is not yet sufficient evidence to rule out contributions from other chemicals. Vaporization of this ester produces toxic pyrolysis products.










