Sodium Chloride
Salt. Used to increase the viscosity in some cosmetics. Can cause eye and skin irritation if used in too high concentrations.
Sodium chloride /ˌsoʊdiəm ˈklɔːraɪd/, commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g/mol respectively, 100 g of NaCl contains 39.34 g Na and 60.66 g Cl. Sodium chloride is the salt most responsible for the salinity of seawater and of the extracellular fluid of many multicellular organisms. In its edible form, salt (also known as table salt) is commonly used as a condiment and food preservative. Large quantities of sodium chloride are used in many industrial processes, and it is a major source of sodium and chlorine compounds used as feedstocks for further chemical syntheses. Another major application of sodium chloride is de-icing of roadways in sub-freezing weather.
 Sodium chloride crystals in a form of halite
| |
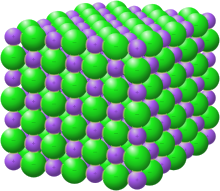 Crystal structure with sodium in purple and chloride in green
| |
| Names | |
|---|---|
| IUPAC name
Sodium chloride
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3534976 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.726 |
| EC Number |
|
| 13673 | |
| KEGG | |
| MeSH | Sodium+chloride |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| NaCl | |
| Molar mass | 58.443 g/mol |
| Appearance | Colorless cubic crystals |
| Odor | Odorless |
| Density | 2.17 g/cm3 |
| Melting point | 800.7 °C (1,473.3 °F; 1,073.8 K) |
| Boiling point | 1,465 °C (2,669 °F; 1,738 K) |
| 360 g/1000 g pure water at T = 25 °C | |
| Solubility in ammonia | 21.5 g/L at T = ?[clarification needed] |
| Solubility in methanol | 14.9 g/L at T = ?[clarification needed] |
| −30.2·10−6 cm3/mol | |
Refractive index (nD)
|
1.5441 (at 589 nm) |
| Structure | |
| Face-centered cubic (see text), cF8 | |
| Fm3m (No. 225) | |
a = 564.02 pm
| |
Formula units (Z)
|
4 |
| octahedral at Na+ octahedral at Cl− | |
| Thermochemistry | |
Heat capacity (C)
|
50.5 J/(K·mol) |
Std molar
entropy (S⦵298) |
72.10 J/(K·mol) |
Std enthalpy of
formation (ΔfH⦵298) |
−411.120 kJ/mol |
| Pharmacology | |
| A12CA01 (WHO) B05CB01 (WHO), B05XA03 (WHO), S01XA03 (WHO) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3 g/kg (oral, rats) |
| Related compounds | |
Other anions
|
Sodium fluoride Sodium bromide Sodium iodide Sodium astatide |
Other cations
|
Lithium chloride Potassium chloride Rubidium chloride Caesium chloride Francium chloride |
| Supplementary data page | |
| Sodium chloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |










