Salicylic Acid
Salicylic acid is a type of beta hydroxy acid that can be found naturally in nature or manufactured synthetically and used in skin treatment products. If you have ever had issues with acne, blackheads, whiteheads, or enlarged pores, then you are likely familiar with salicylic acid (SA). Even if you have not heard of it, you have probably used it, as it is the go-to solution for these types of skin problems, together with benzoyl peroxide. SA is an ingredient that is soluble in lipids, which means it can penetrate the pores of those who have oily or acne-prone skin, particularly those with “open comedones,” better known as blackheads. This makes it especially effective since it helps to keep the pores free of any substances that can cause acne.
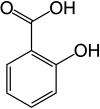
Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substances Control Act (TSCA) Chemical Substance Inventory as an experimental teratogen. The name is from Latin salix for willow tree. It is an ingredient in some anti-acne products. Salts and esters of salicylic acid are known as salicylates.
|
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Hydroxybenzoic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.648 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.122 g/mol | ||
| Appearance | Colorless to white crystals | ||
| Odor | Odorless | ||
| Density | 1.443 g/cm3 (20 °C) | ||
| Melting point | 158.6 °C (317.5 °F; 431.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) decomposes 211 °C (412 °F; 484 K) at 20 mmHg | ||
| Sublimes at 76 °C | |||
| |||
| Solubility | Soluble in ether, CCl4, benzene, propanol, acetone, ethanol, oil of turpentine, toluene | ||
| Solubility in benzene |
| ||
| Solubility in chloroform |
| ||
| Solubility in methanol |
| ||
| Solubility in olive oil | 2.43 g/100 g (23 °C) | ||
| Solubility in acetone | 39.6 g/100 g (23 °C) | ||
| log P | 2.26 | ||
| Vapor pressure | 10.93 mPa | ||
| Acidity (pKa) |
| ||
| UV-vis (λmax) | 210 nm, 234 nm, 303 nm (4 mg/dL in ethanol) | ||
| −72.23·10−6 cm3/mol | |||
Refractive index (nD)
|
1.565 (20 °C) | ||
| 2.65 D | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
−589.9 kJ/mol | ||
Std enthalpy of
combustion (ΔcH⦵298) |
3.025 MJ/mol | ||
| Pharmacology | |||
| A01AD05 (WHO) B01AC06 (WHO) D01AE12 (WHO) N02BA01 (WHO) S01BC08 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Eye hazards
|
Severe irritation | ||
Skin hazards
|
Mild irritation | ||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H302, H318 | |||
| P280, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 157 °C (315 °F; 430 K) closed cup | ||
| 540 °C (1,004 °F; 813 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
480 mg/kg (mice, oral) | ||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Related compounds
|
Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid, Salicylate synthase | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||