Phenoxyethanol
Phenoxyethanol is a widely used synthetic preservative in skin care products and also as a stabilizer in perfumes and soaps. It generally has global approval for us in cosmetic products in concentrations up to 1%. It is often used in lower percentages when coupled with another preservative or preservative boosting ingredient such as ethylhexylglycerin.
Phenoxyethanol is very versatile as it’s effective and stable at various pH ranges and has a broad spectrum activity against many pathogens. It is stable and it’s compatible with many other preservatives used in cosmetics.
In late 2019, concerns began to surface regarding some reactions to Phenoxyethanol and some small studies that indicate it can be contaminated with carcinogenic compounds. The allergic reactions and dire warnings are somewhat unfounded, as these issues occur when significantly higher concentrations of phenoxyethanol are present – far above the 1% safe levels used in cosmetics. Regardless, concerns over phenoxyethanol have the cosmetic industry searching for new preservative solutions – though phenoxyethanol is still considered safe – and it’s use is far safer and more preferable to cosmetics growing bacteria or mold.
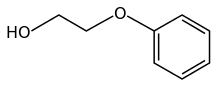
Phenoxyethanol is the organic compound with the formula C6H5OC2H4OH. It is a colorless oily liquid. It can be classified as a glycol ether and a phenol ether. It is a common preservative in vaccine formulations.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Phenoxyethan-1-ol | |
| Other names
Phenoxyethanol
Ethylene glycol monophenyl ether Phenoxytolarosol Dowanol EP / EPH Protectol PE Emery 6705 Rose ether 1-Hydroxy-2-phenoxyethane β-hydroxyethyl phenyl ether Phenyl cellosolve | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.173 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10O2 | |
| Molar mass | 138.166 g·mol−1 |
| Appearance | Colorless oily liquid |
| Odor | faint rose-like |
| Density | 1.102 g/cm3 |
| Melting point | −2 °C (28 °F; 271 K) |
| Boiling point | 247 °C (477 °F; 520 K) |
| 26 g/kg | |
| Solubility | Chloroform, Alkali, diethyl ether: soluble |
| Solubility in peanut oil | slightly |
| Solubility in olive oil | slightly |
| Solubility in acetone | miscible |
| Solubility in ethanol | miscible |
| Solubility in glycerol | miscible |
| Vapor pressure | 0.001 kPa (0.00015 psi) |
| Thermal conductivity | 0.169 W/(m⋅K) |
Refractive index (nD)
|
1.534 (20 ℃) |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful if swallowed Causes serious eye irritation |
| GHS labelling: | |

| |
| Warning | |
| NFPA 704 (fire diamond) | |
| Flash point | 126 °C (259 °F; 399 K) |
| 430 °C (806 °F; 703 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1850 mg/kg (rat, oral) |
| Related compounds | |
Related compounds
|
phenetole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |










