Naringenin
Naringenin is a naturally occurring flavonoid found predominantly in citrus fruits like grapefruit, oranges, and lemons. As a bioactive compound, it has garnered attention in skincare and health due to its antioxidant, anti-inflammatory, and antimicrobial properties. Structurally, naringenin belongs to the flavanone class, characterized by its ability to neutralize free radicals and protect cells from oxidative damage.
In skincare, naringenin is celebrated for its ability to reduce redness, soothe irritated skin, and enhance skin’s resilience against environmental stressors. Its antioxidant capabilities also make it effective in combating premature aging, while its antimicrobial properties can help maintain a clearer complexion by reducing the presence of harmful bacteria.
Naringenin is commonly incorporated into formulations aimed at brightening the skin, calming inflammation, and protecting against external pollutants. Its natural origins and multifunctionality make it a preferred choice for those seeking gentle yet effective skincare solutions.
Naringenin is a flavanone from the flavonoid group of polyphenols. It is commonly found in citrus fruits, especially as the predominant flavonone in grapefruit.
| |

| |
| Names | |
|---|---|
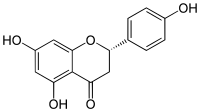
| IUPAC name
(2S)-4′,5,7-Trihydroxyflavan-4-one
| |
| Systematic IUPAC name
(2S)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Naringetol; Salipurol; Salipurpol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.865 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H12O5 | |
| Molar mass | 272.256 g·mol−1 |
| Melting point | 251 °C (484 °F; 524 K) |
| 475 mg/L[citation needed] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The fate and biological functions of naringenin in vivo are unknown, remaining under preliminary research, as of 2024. High consumption of dietary naringenin is generally regarded as safe, mainly due to its low bioavailability. Taking dietary supplements or consuming grapefruit excessively may impair the action of anticoagulants and increase the toxicity of various prescription drugs.
Similar to furanocoumarins present in citrus fruits, naringenin may evoke CYP3A4 suppression in the liver and intestines, possibly resulting in adverse interactions with common medications.









