L-Arginine
This essential amino acid, known as L-arginine or arginine, is naturally found in the human body and is a significant component of proteins. As a vital element for human well-being, it has shown to have antioxidant properties and can help repair damaged skin. Furthermore, it has been extensively explored for its hydrating properties, as it assists in the skin’s natural production of substances such as proline and urea. Both animal-derived and synthetic types of arginine are equally effective when it comes to skin care benefits.
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid.
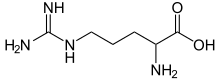 Skeletal formula of arginine
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Arginine
| |||
| Other names
2-Amino-5-guanidinopentanoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet |
| ||
| 1725411, 1725412 D, 1725413 L | |||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| DrugBank |
| ||
| ECHA InfoCard | 100.000.738 | ||
| EC Number |
| ||
| 364938 D | |||
| |||
| KEGG |
| ||
| MeSH | Arginine | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H14N4O2 | |||
| Molar mass | 174.204 g·mol−1 | ||
| Appearance | White crystals | ||
| Odor | Odourless | ||
| Melting point | 260 °C; 500 °F; 533 K | ||
| Boiling point | 368 °C (694 °F; 641 K) | ||
| 14.87 g/100 mL (20 °C) | |||
| Solubility | slightly soluble in ethanol insoluble in ethyl ether | ||
| log P | −1.652 | ||
| Acidity (pKa) | 2.18 (carboxyl), 9.09 (amino), 13.8 (guanidino) | ||
| Thermochemistry | |||
Heat capacity (C)
|
232.8 J K−1 mol−1 (at 23.7 °C) | ||
Std molar
entropy (S⦵298) |
250.6 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−624.9–−622.3 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−3.7396–−3.7370 MJ mol−1 | ||
| Pharmacology | |||
| B05XB01 (WHO) S | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H319 | |||
| P305+P351+P338 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
5110 mg/kg (rat, oral) | ||
| Safety data sheet (SDS) | L-Arginine | ||
| Related compounds | |||
Related alkanoic acids
|
|||
Related compounds
|
|||
| Supplementary data page | |||
| Arginine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||