Carbomer
A popular and widely used ingredient, a polymer of acrylic acid, it’s a large molecule formed by repeating subunits that has the ability to transform a liquid into a pleasant gel formula. In order for thickening to occur, it often requires neutralization with a base like sodium hydroxide. The result is a clear, viscous gel that feels comfortable and non-sticky on the skin. Typically, it is used in small quantities, usually 1% or less, in most formulations.
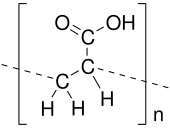
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications.
| |
| Names | |
|---|---|
| IUPAC name
Poly(acrylic acid), poly(1-carboxyethylene)
| |
| Other names
PAA, PAAc, Acrysol, Acumer, Alcosperse, Aquatreat, Carbomer, Sokalan
| |
| Identifiers | |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.115.375 |
| EC Number |
|
| KEGG | |
| UNII |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C3H4O2)n | |
| Molar mass | variable |
| log P | 0.25700 |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |










