Ascorbic Acid
Ascorbic acid, also known as L-ascorbic acid, is a well researched skin care ingredient. This powerful antioxidant naturally occurs in abundance in your skin. When used for skin care in concentrations below 2% it helps to improve the appearance of uneven skin tone, fine lines, and imparts its antioxidant action. At higher concentrations, of 5% and above, it offers enhanced benefits. However, this pure Vitamin C ingredients, is highly reactive to air, light, and requires pH levels of 3.5 and lower to remain stable. It’s why high concentrations of Ascorbic Acid are expensive. And it’s why other more stable forms of vitamin c are often used in skin care formulas.
You’ll often see Ascorbic Acid combined with other antioxidants, such as vitamin E and ferulic acid, to boost it’s overall effectiveness and to stabilize the vitamin c in solution.
Vitamin C (also known as ascorbic acid and ascorbate) is a water-soluble vitamin found in citrus and other fruits and vegetables, also sold as a dietary supplement and as a topical 'serum' ingredient to treat melasma (dark pigment spots) and wrinkles on the face. It is used to prevent and treat scurvy. Vitamin C is an essential nutrient involved in the repair of tissue, the formation of collagen, and the enzymatic production of certain neurotransmitters. It is required for the functioning of several enzymes and is important for immune system function. It also functions as an antioxidant. Most animals are able to synthesize their own vitamin C. However, apes (including humans) and monkeys (but not all primates), most bats, some rodents, and certain other animals must acquire it from dietary sources.
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /əˈskɔːrbɪk/, /əˈskɔːrbeɪt, -bɪt/ |
| Trade names | Ascor, Cevalin, others |
| Other names | l-ascorbic acid, ascorbic acid, ascorbate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682583 |
| License data | |
| Routes of administration | By mouth, intramuscular (IM), intravenous (IV), subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Rapid and complete |
| Protein binding | Negligible |
| Elimination half-life | Varies according to plasma concentration |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| NIAID ChemDB | |
| PDB ligand | |
| E number | E300 (antioxidants, ...) |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.061 |
| Chemical and physical data | |
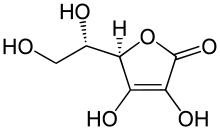
| Formula | C6H8O6 |
| Molar mass | 176.124 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.694 g/cm3 |
| Melting point | 190 to 192 °C (374 to 378 °F) (some decomposition) |
| Boiling point | 552.7 °C (1,026.9 °F) |
| |
| |
| (verify) | |
There is some evidence that regular use of supplements may reduce the duration of the common cold, but it does not appear to prevent infection. It is unclear whether supplementation affects the risk of cancer, cardiovascular disease, or dementia. It may be taken by mouth or by injection.
Vitamin C is generally well tolerated. Large doses may cause gastrointestinal discomfort, headache, trouble sleeping, and flushing of the skin. Normal doses are safe during pregnancy. The United States Institute of Medicine recommends against taking large doses.
Vitamin C was discovered in 1912, isolated in 1928, and, in 1933, was the first vitamin to be chemically produced. It is on the World Health Organization's List of Essential Medicines. Vitamin C is available as an inexpensive generic and over-the-counter medication. Partly for its discovery, Albert Szent-Györgyi and Walter Norman Haworth were awarded the 1937 Nobel Prizes in Physiology and Medicine and Chemistry, respectively. Foods containing vitamin C include citrus fruits, kiwifruit, guava, broccoli, Brussels sprouts, bell peppers, potatoes, and strawberries. Prolonged storage or cooking may reduce vitamin C content in foods.









