Alkalinity
Solutions that have a pH of above 7.0. Most natural soaps and shampoos have a pH of about 8.0 to 9.0. “pH balanced” products are sold with the claim that the lower pH of around 4.5 is better for the hair and skin. However, it is important for soaps and shampoos to have a natural mild alkalinity in order to clean and destroy the bacteria that cause diseases like impetigo.
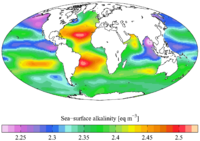
Alkalinity (from Arabic: القلوي, romanized: al-qaly, lit. 'ashes of the saltwort') is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale.
Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant.
Although alkalinity is primarily a term used by oceanographers, it is also used by hydrologists to describe temporary hardness. Moreover, measuring alkalinity is important in determining a stream's ability to neutralize acidic pollution from rainfall or wastewater. It is one of the best measures of the sensitivity of the stream to acid inputs. There can be long-term changes in the alkalinity of streams and rivers in response to human disturbances such as acid rain generated by SOx and NOx emissions.